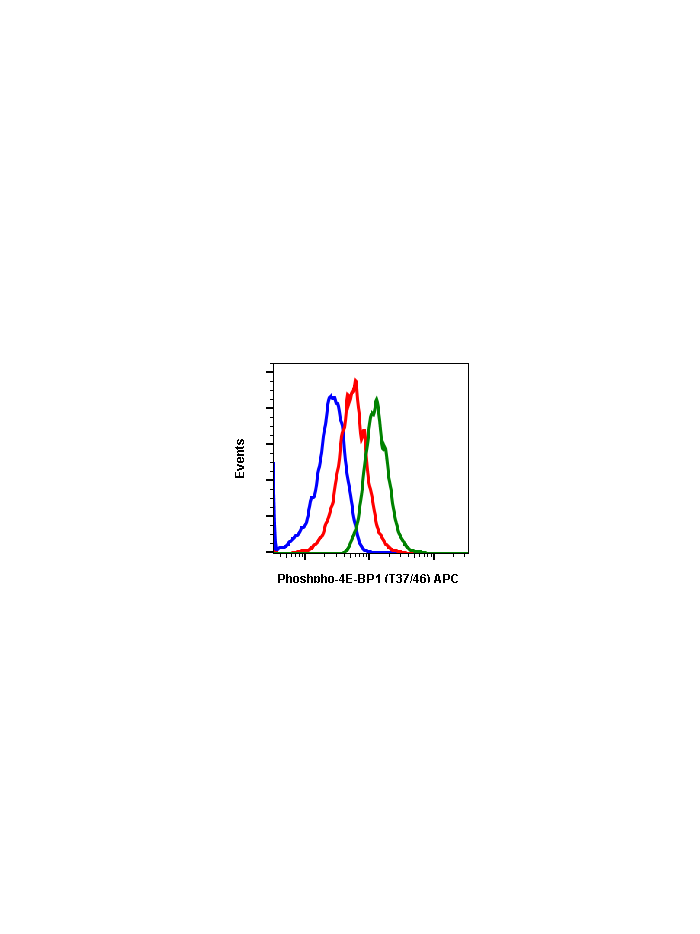
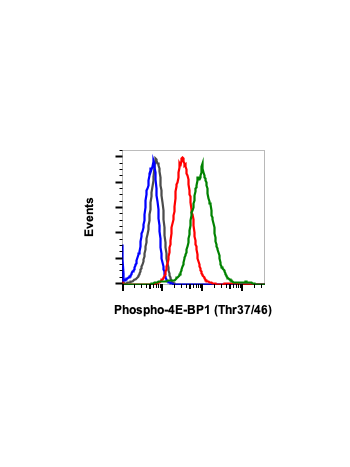
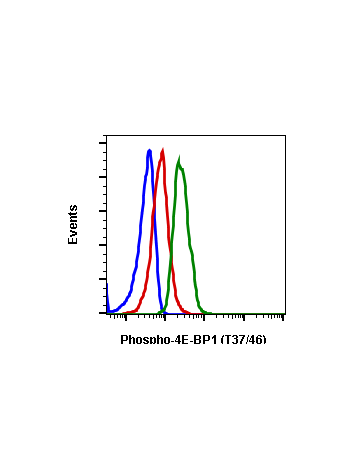
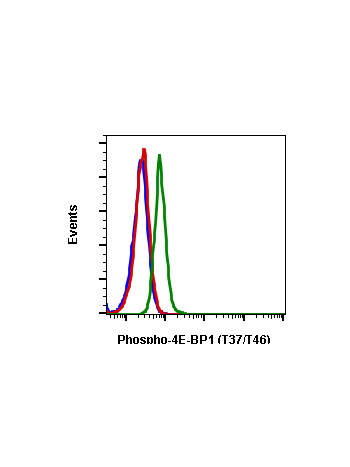
Phospho-4E-BP1 (Thr37/46) (A5) rabbit mAb APC conjugate
From
$99.00
In stock
Only %1 left
SKU
2044
4E-BP1 regulates protein translations induced by upstream signals initiated by ERK1/2 MAP kinase or PI3-Kinase/AKT/mTOR pathways. Ultimately activation of mTOR, positively stimulates mRNA translation by its two dominant downstream substrates: ribosomal protein S6 kinase, and S6K1. Translation of mammalian mRNA is directed through cap dependent translation; the capping of 5’ end of mRNA by m7-GTP that allows recruitment of eIF4F complex and the binding of 40S ribosomal subunit to the 5’ mRNA cap. eIF4E is a heterotrimeric protein composed of the DEAD-box RNA helicase eIF4A, the cap binding eIF4E and the large scaffolding protein eIF4G. 4E-BP competes with eIF4G for binding to the same conserved patch of hydrophobic residues on the dorsal side of the eIF4E thus acting as a molecular mimic. Activated 4E-BP1 induces rapid cessation of cap dependent translation by binding to the translation factor eIF4E and preventing its interaction with eIF4G, thus inhibiting the pre-initiation complex formation. 4E-BP1 activity is regulation by multiple post-translational modification. Seven phosphorylation residues have been discovered in human 4E-BP1 including Thr37, Thr46, Ser56, Thr70, Ser83, Ser101, and Ser112. 4E-BP1 phosphorylation is up-regulated by extracellular stimuli, including serum, hormones, cytokines, and G-protein coupled receptor agonists while starvation for essential nutrients, growth factor deprivation, ischemia, hypoxia, and ethanol toxicity, strenuous exercise, exposure to glucocorticoids, and infection. Phosphorylation of 4E-BP1 regulates 4E-BP1 binding and downstream regulation. Binding of 4E-BP1 to proteins containing 4E containing domains reduce translation of specific mRNAs which include prosurvival factor Mcl-1, cell cycle regulator Cyclin D3, pro-angiogenic growth factor VEGF among others. In addition, 4E-BP competes with eIF4G proteins for a common binding site on the cap-binding protein, eIF4E. 4E-BP1 binding to eIF4E is highly modulated by the degree of 4E-BP1 phosphorylation. Hyperphosphorylation of 4E-BP1 by various extracellular stimuli activates decreases its affinity to eIF4E and hence eIF4E release. In turn eIF4E has an opportunity to bind to eIF4G forming function eIF4F complex. On the other hand, hypo-phosphorylation of 4E-BP1 increases 4E-BP1 affinity for eIF4E, inhibiting eIF4F formation.
| Applications | Flow Cytometry |
|---|---|
| Clone | 4EB1T37T46-A5 |
| Format | APC |
| Validated Reactivity | Human, Mouse, Rat |
| Cross Reactivity | Predicted to work with mouse, rat, and other homologues. |
| Clonality | Monoclonal |
| Immunogen | A synthetic phospho-peptide corresponding to residues surrounding Thr37/46 of human phospho 4E-BP1 |
| Formulation | 1X PBS, 0.09% NaN3, 0.2% BSA |
| Isotype | Rabbit IgGk |
| Preparation | Protein A+G |
| Recommended Usage | For flow cytometric staining, the suggested use of this reagent is 5 µL per million cells or 5 µL per 100 µL of staining volume. It is recommended that the reagent be titrated for optimal performance for each application. |
| Storage | 2-8ºC |
| Pseudonyms | Eukaryotic translation initiation factor 4E-binding protein 1, eIF4E-binding protein 1, PHAS-I, EIF4EBP1 |
| Uniprot ID | Q13541 |
| References | Hay N. Sonenberg N, (2004) Gens Dev. 18: 1926-1945. Aitken CE, and Lorsch JR, (2012), Nat Struc Mol Biol 19 : 568-576. Peter D., et al., (2015) Mol Cell, 57:1074-1087. Pain VM, (1996) Eur J Biochem 236: 747-771. Fadden P, Haystead TA, Lawrence Jr JC, (1997) J Biol Chem 272: 10240-10247. Kimball SR et al., (1999) J Biol Chem 274: 11647-1152. Kleijn M and Proud CG, (2000) Biochem J, 347: 399-406. Lang CH et al., (2000) Clin Exp Res 24: 322-331. Shah OJ, Kimbell SR., Jefferson LS., (2000), Am J Physiol Endocrinol Metab 278: E76-E82. |
Write Your Own Review