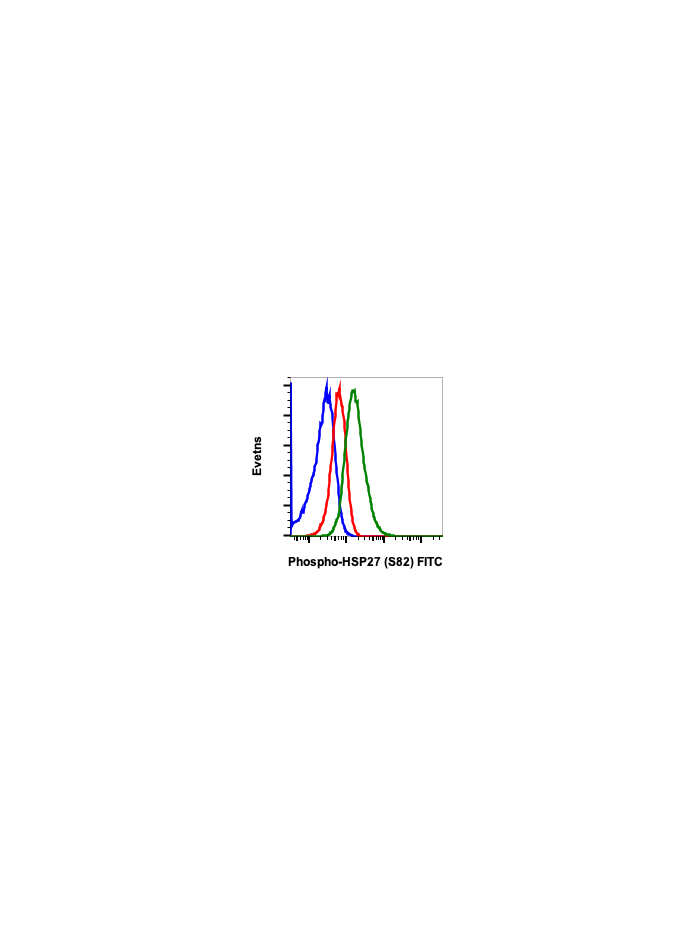
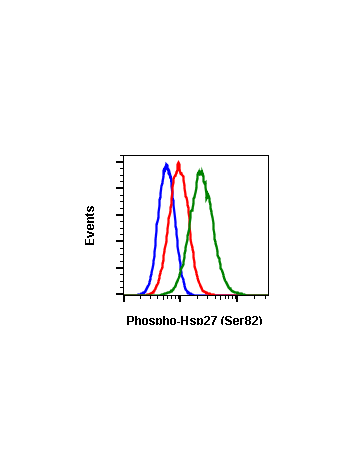
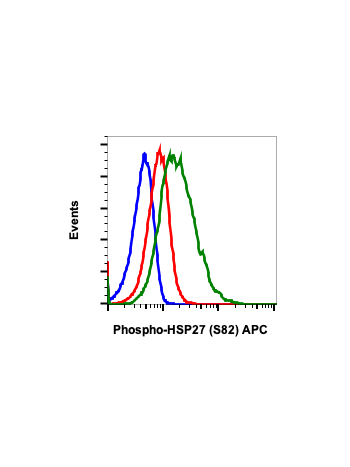
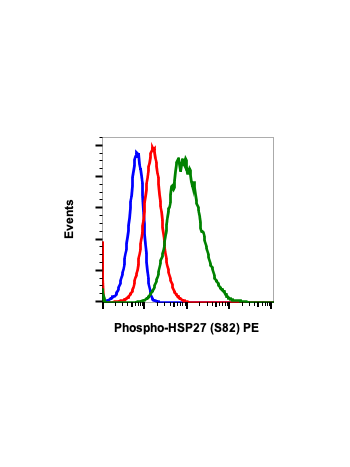
Phospho-HSP27 (Ser82) (CB2) rabbit mAb FITC conjugate
Human Hsp27 (also called HspB) is a heat shock protein whose level is upregulated when cells are exposed to conditions that alter protein folding. Hsp27 is an ATP-independent molecular chaperone involved in the protein refolding machinery (1). It functions as a tool to trap and store stress-induced mis-folded polypeptides to avoid their aggregation and to indirectly promote their refolding or proteolytic degradation (2,3). Hsp27 is also known for its control of cytoskeletal organization (4-6) and for its antiapoptotic and antioxidant properties (7-9).
Hsp27 phosphorylation level could be modified by agents such as phorbol ester, calcium ionophores, or stress conditions such as heat shock (10). Human Hsp27 contains three phosphorylatable serine sites (Ser15, Ser78, and Ser82) located in its N-terminal domain whereas murine Hsp27, also called Hsp25, has only two phosphorylated serine sites (Ser15 and Ser86). Several kinases phosphorylate Hsp27 (11) including mitogen-activated protein kinases associated protein kinases (MAPK/MK2,3), MK5-PRAK, PKCγ, and PKD. Moreover, threonine 143 (Thr143) of human Hsp27 appears to be phosphorylated by cGMP-dependent protein kinase. Therefore, Hsp27 often displays complex patterns of phosphorylation since several signaling pathways, including those induced by stress, mitogen, and differentiation, activate these kinases. Hsp27 is involved with cell differentiation (12). It also modifies apoptotic pathways through upstream and downstream of mitochondria by decreasing activity of caspase 9 and caspase 3 (13,14). These events are activated in heat shock, oxidative stress, and endoplasmic reticulum stress-induced apoptosis (15, 16).
Hsp27 Phosphorylation:
Recently, it has been shown that Sec6 is involved with Hsp27 Ser78, and Ser82 phosphorylation through p38 MAPK promoting cell migration and inhibition of apoptosis induced by TNFa(17). MAPKAPK2 (MK2)/Hsp27 axis have been shown to promote tumorigenesis through phosphorylation of Hsp27 by MK2 (18). Hsp27 (Ser78/82) phosphorylation through PKCa (Thr638), p38MAPK (Thr180/Tyr182) is elevated by chemokine CC-motif ligand 2 (CCL2) in platelets suggesting that PKCa/pMAPK/Hsp27 axis may be target for antiplatelet drugs and the treatment for cardiovascular diseases (19).
| Applications | Flow Cytometry |
|---|---|
| Clone | HSP27S82-CB2 |
| Format | FITC |
| Validated Reactivity | Human, Mouse |
| Cross Reactivity | Predicted to work with mouse, rat and other homologues. |
| Clonality | Monoclonal |
| Immunogen | A synthetic phospho-peptide corresponding to residues surrounding Ser82 of human phospho Hsp27 |
| Formulation | 1X PBS, 0.09% NaN3, 0.2% BSA |
| Isotype | Rabbit IgGk |
| Preparation | Protein A+G |
| Recommended Usage | For flow cytometric staining, the suggested use of this reagent is 5 µL per million cells or 5 µL per 100 µL of staining volume. It is recommended that the reagent be titrated for optimal performance for each application. See product image legends for additional information. |
| Storage | 2-8ºC |
| Pseudonyms | Heat shock protein beta-1, HspB1, 28 kDa heat shock protein, Estrogen-regulated 24 kDa protein, Heat shock 27 kDa protein, Stress-responsive protein 27, SRP27, HSP28 |
| Uniprot ID | P04792 |
| References | 1. Jakob U, et al. (1993) J Biol Chem. 268:1517–20. 2. Ehrnsperger M, et al. (1999) J Biol Chem. 274:14867–74. 3. Ehrnsperger M, et al. (2000) Methods Mol Biol. 99:421–9. 4. Dalle-Donne I, et al. (2001) Free Radic Biol Med. 31:1624–32. 5. Mounier N, et al. (2002) Cell Stress Chaperones 7:167–76. 6. Wettstein G, et al. (2012) Int J Biochem Cell Biol 44:1680–6. 7. Arrigo A-P, et al. (2005) Methods 35:126–38. 8. Bruey JM, et al. (2000) Nat Cell Biol 2:645–52. 9. Preville X, et al. (1999) Exp Cell Res 247:61–78. 10. Welch WJ. (1985) J Biol Chem 260:3058–62. 11. Kostenko S, & Moens U. (2009) Cell Mol Life Sci 66:3289–07. 12. Davidson SM, & Morange M. (2000) Dev Biol 218:146–60. 13. Garrido C, et al. (1999) FASEB J. 1999;13:2061–70. 14. Pandey P, at al. (2000) Oncogene 19:1975–81. 15. Zhang H aet al. (2017) J cell Biochem Dec 12. Doi: 10.1002/jcb.26575. 16. Kennedy D at al. (2017) Cll Death Dis Aug 31:8(8)e3026. 17. Tanaka T. et al. (2018) Cell Signaling 49:1-16. 18. Henriques A. El a. (2018) Proc Natl Acad Sci U.S.A. 115(24)E5546-55. 19. Liu D. (2018) Biochim Biophys Acta, June 1. Pii: S0925-4439. |