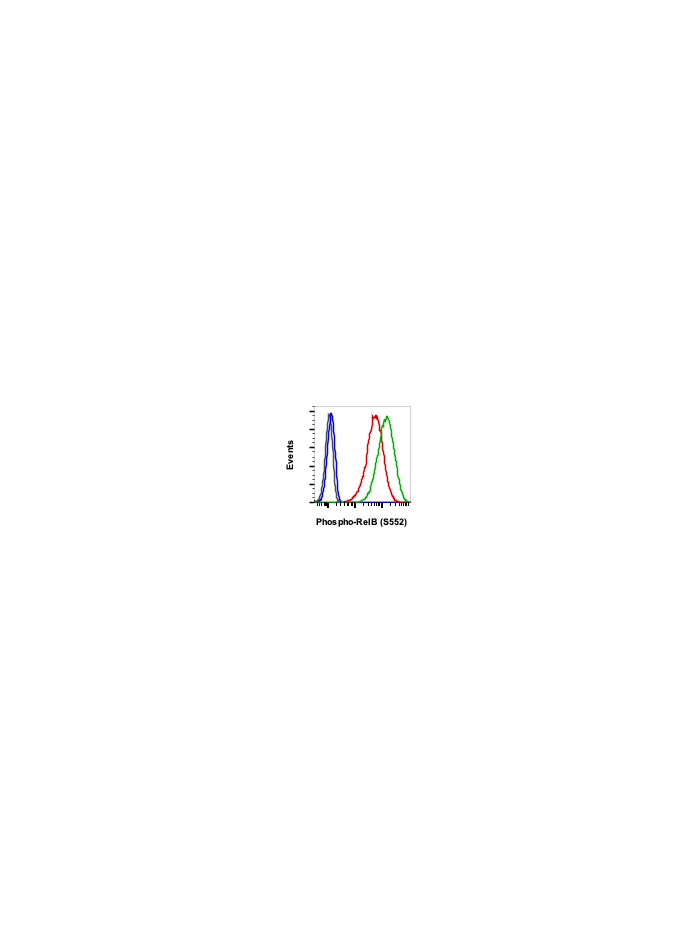
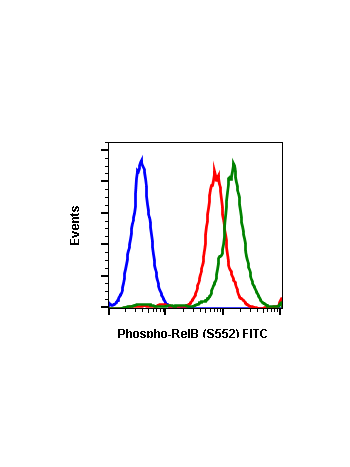
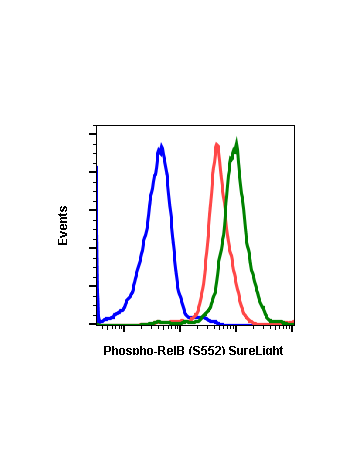
Phospho-RelB (Ser552) (A7) rabbit mAb
From
$210.00
In stock
Only %1 left
SKU
2206
RelB contains the Rel homology Domain (RHD) shared by all members of the NF-kB family (1). It is best known for its roles in lymphoid development, DC biology, and noncanonical signaling (2). RelB is a major contributor to chromatin biology, frequently functioning as a dual transcription factor that silences sets of genes by generating silent facultative heterochromatin and activates euchromatin of others (3). RelB is required to repress immediate-response proinflammatory genes during endotoxin tolerance (4). The N-terminal leucine zipper motif of RelB, a motif unique among the NF-κB family, may associate with more diverse DNA sequences than other NF-κB members (5). RelB binds to DNA but only after forming a heterodimer with NF-kB p50 or p52 (6).
Like all NF-kB members, RelB contains an ∼300-residue region known as the RHD. This region supports many of the NF-kB essential functions, such as DNA binding, dimerization, and nuclear localization (7). RelB, unlike other NF-kB members, has an N-terminal leucine zipper motif (1), a domain that can typically interact with many proteins (8). p100, the C-terminus of NF-kB2 acts as a RelB inhibitor (9), where it sequesters RelB in the cytosol to repress its activity. RelB is phosphorylated rapidly at threonine 84 and serine 552, causing cleavage at the N-terminus and its degradation by the proteasome (10). These phosphorylations are mediated by glycogen synthase kinase-3β, specific inhibition of which blocks phospho RelB phosphorylation and degradation (11). This degradation does not occur when phospho RelB is phosphorylated at serine 368, allowing its association with and stabilization by p100 (12).
Like all NF-kB members, RelB contains an ∼300-residue region known as the RHD. This region supports many of the NF-kB essential functions, such as DNA binding, dimerization, and nuclear localization (7). RelB, unlike other NF-kB members, has an N-terminal leucine zipper motif (1), a domain that can typically interact with many proteins (8). p100, the C-terminus of NF-kB2 acts as a RelB inhibitor (9), where it sequesters RelB in the cytosol to repress its activity. RelB is phosphorylated rapidly at threonine 84 and serine 552, causing cleavage at the N-terminus and its degradation by the proteasome (10). These phosphorylations are mediated by glycogen synthase kinase-3β, specific inhibition of which blocks phospho RelB phosphorylation and degradation (11). This degradation does not occur when phospho RelB is phosphorylated at serine 368, allowing its association with and stabilization by p100 (12).
| Applications | Flow Cytometry |
|---|---|
| Clone | RelBS552-A7 |
| Format | Unconjugated |
| Validated Reactivity | Human, Mouse |
| Cross Reactivity | Predicted to work with mouse, rat and other homologues. |
| Detection | Anti-Rabbit IgG |
| Clonality | Monoclonal |
| Immunogen | A synthetic phospho-peptide corresponding to residues surrounding Ser552 of human phospho RelB |
| Formulation | 1X PBS, 0.02% NaN3, 50% Glycerol, 0.1% BSA |
| Isotype | Rabbit IgGk |
| Preparation | Protein A+G |
| Recommended Usage | 1µg/mL – 0.001µg/mL. It is recommended that the reagent be titrated for optimal performance for each application. See product image legends for additional information. |
| Storage | -20ºC |
| Pseudonyms | I-Rel |
| Uniprot ID | Q01201 |
| References | 1. Ryseck R P et al., (1992), Cell. Biol. 12, 674–684. 2. Vallabhapurapu S., and Karin M. (2009), Annu. Rev. Immunol. 27, 693–733. 3. McCall C E and Yoza B K, (2007) Am. J. Respir. Crit. Care Med. 175, 763–767. 4. Yoza B K et al., (2006) J. Immunol. 177, 4080–4085. 5. Liu T F et al., (2011) J. Biol. Chem. 286, 9856–9864. 5. Moorthy AK et al., (2007) J. Mol. Biol. 373, 723–734. 6. Bours V et al., (1994) Oncogene 9, 1699–1702. 7. Baldwin A. S., Jr., (1996) Annu. Rev. Immunol. 14, 649–683. 8. Alber T. (1992), Curr. Opin. Genet. Dev. 2, 205–210. 9. Dobrzanski P et al., (1993), Mol. Cell. Biol. 13, 1572–1582. 10 Marienfeld R et al., (2001), Oncogene 20, 8142–8147. 11. Neumann M. Et al., (2011), Oncogene 30, 2485–2492. 12 Maier HJ et al., (2003), J. Biol. Chem. 278, 39242–39250. |
Write Your Own Review